FORENSIC ENGINEERING AND EXPERT WITNESS SERVICES
3 November 2022
What are the Sources of Hydrogen That Are Used for Power Generation?
There are many ways to generate electrical power on an industrial scale, but one of the sources that are becoming more prevalent these days is hydrogen. In today’s era, some 4% of all energy worldwide is generated from hydrogen, and this percentage is growing as hydrogen can be relatively cheaply sourced.
There are four primary sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis, which account for 48%, 30%, 18%, and 4% of the world’s hydrogen production, respectively. Fossil fuels are the dominant source of industrial hydrogen, accounting for between 95% and 98% of global supplies. Carbon dioxide can be separated from natural gas for hydrogen production with a 70-85% efficiency and from other hydrocarbons at varying degrees of efficiency.
Specifically, the six most common methods for producing hydrogen are:
• Steam Reforming of Natural Gas
• Partial Oxidation of Other Hydrocarbons
• Coal Gasification
• Electrolysis of Water
• Biomass Gasification
• Methane Pyrolysis
The details of these methods are as follows:
Steam Reforming of Natural Gas
Steam reforming of natural gas is a process by which the methane in natural gas is reacted with steam at high temperatures (700-1100°C) to produce hydrogen and carbon dioxide. Steam reforming is one of the most common processes used to produce hydrogen, accounting for nearly half of the world’s hydrogen supply. It’s also the most energy-efficient process for hydrogen production. The process is highly endothermic and requires a catalyst; the most common catalyst that’s used is nickel.
The steam reforming reaction can be expressed as:
CH_4 + H_2O → CO_2 + 3H_2
Partial Oxidation of Other Hydrocarbons
Partial oxidation is a process by which hydrocarbons are partially oxidized at high temperatures (700-1100°C) and pressures (1-30 bar) to produce hydrogen and carbon monoxide. The most common feedstock for partial oxidation is methane, but other hydrocarbons, such as propane, butane, and naphtha, can also be used.
The partial oxidation reaction can be expressed as follows:
CH_4 + 0.5 O_2 → CO + 2H_2
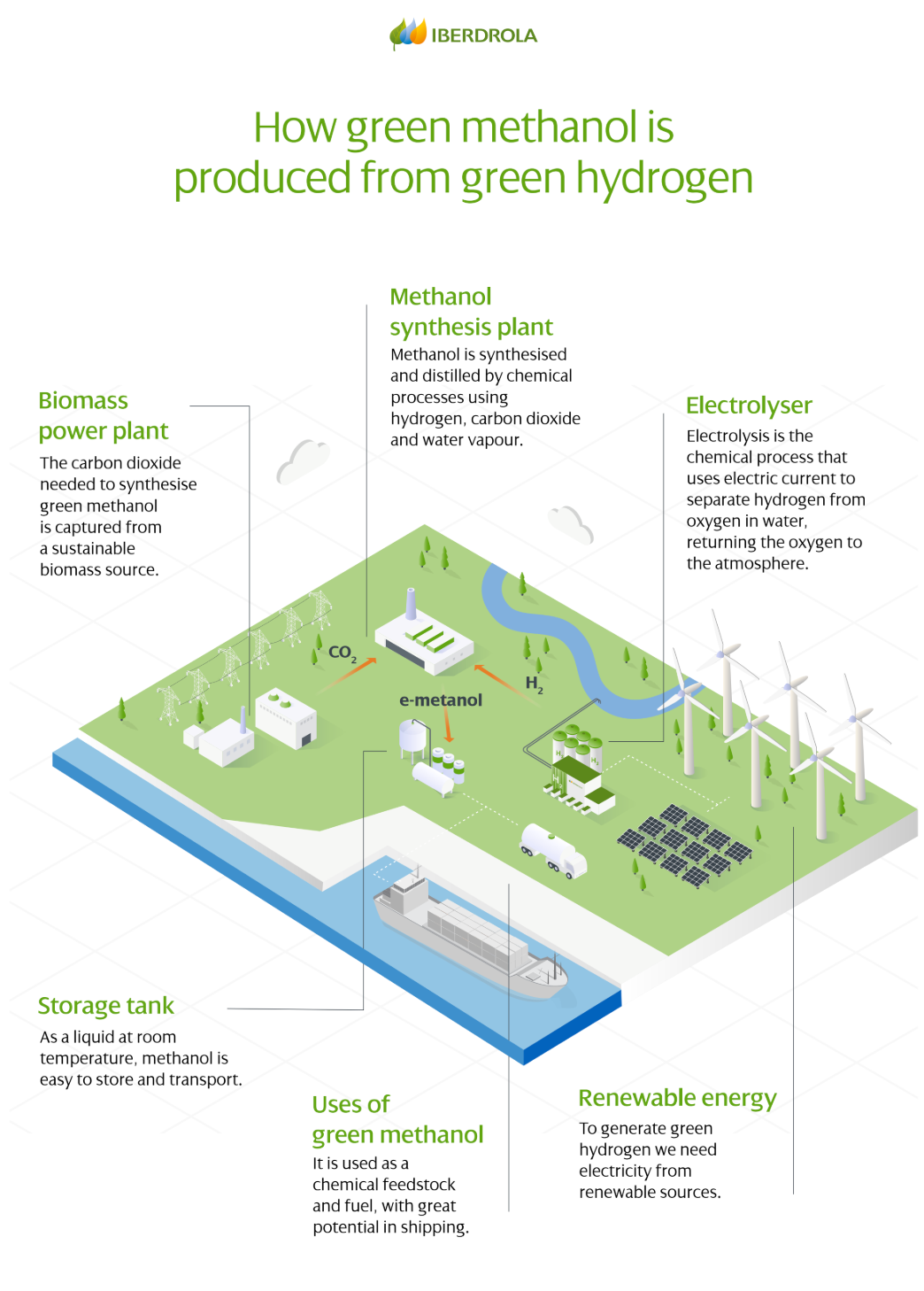
Coal Gasification
Coal gasification is a process by which coal is converted into a gas called synthesis gas, or syngas. Syngas is a mixture of hydrogen, carbon monoxide, carbon dioxide, and other gases. Hydrogen can be separated from the syngas and used for other purposes.
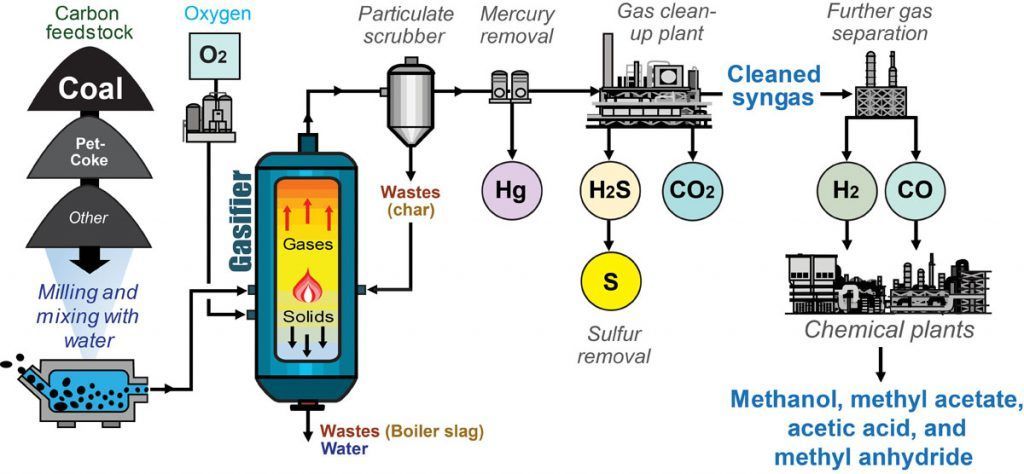
The coal gasification reaction can be expressed as follows:
C + H_2O → CO + H_2
Electrolysis of Water
Electrolysis of water is a process in which water is converted into hydrogen and oxygen by an electric current. The hydrogen can then be used for other purposes. The hydrogen produced by electrolysis is often referred to as “green hydrogen” because it can be produced from renewable energy sources, such as solar and wind power.
The electrolysis of water reaction can be expressed as:
2H_2O → 2H_2 + O_2
Photoelectrochemical water splitting is a process that uses solar energy to drive water electrolysis. In this process, sunlight generates electricity, which drives an electrolyzer. The main advantage of this process is that it’s completely carbon-free. The main disadvantage is that it requires much land to generate the necessary solar power.
The first photoelectrochemical water-splitting demonstration plant was built in the 1970s by the U.S. Department of Energy (DOE). The plant used a parabolic trough solar collector to generate electricity, which, in turn, drove the plant’s electrolyzer. The plant was decommissioned in the early 1990s.
In 2006, the DOE started a project to design and build a new photoelectrochemical water-splitting demonstration plant. This plant will use a photovoltaic array to generate electricity, which will then be used to drive an electrolyzer.
High-temperature electrolysis (HTE) is another type of water electrolysis whereby an electrolyzer is integrated with a nuclear power plant. In this process, nuclear energy is used to generate high-temperature steam, which is used to drive the electrolyzer. A solid oxide fuel cell (SOFC) could also be used instead of an electrolyzer.
The main advantages of HTE are that it can also be completely carbon-free, and the process can be powered by any heat source, including nuclear, solar, or geothermal. The main disadvantage is that it requires very high temperatures, which are difficult to achieve without a nuclear reactor.
The first HTE demonstration plant was built in the 1970s by the U.S. DOE. The plant used a high-temperature gas-cooled reactor to generate steam, which was then used to drive an electrolyzer. This plant was decommissioned in the early 1990s.
Biological water splitting is a third type of water electrolysis, which uses bacteria to split water into hydrogen and oxygen. The bacteria use sunlight to split water into hydrogen and oxygen during this process. Once again, the process is entirely carbon-free. The main disadvantage is that it requires much land to grow the necessary bacteria.
The first biological water-splitting demonstration plant was built in the 1970s by the U.S. DOE. The plant used bacteria to split water into hydrogen and oxygen. This plant was also decommissioned in the early 1990s. In 2006, the U.S. DOE started a project to design and build a new biological water-splitting demonstration plant.
Biomass Gasification
Biomass gasification is a process in which biomass is converted into syngas (in this case, it is sometimes known as biosyngas). The hydrogen can then be separated from the biosyngas and used for other purposes.
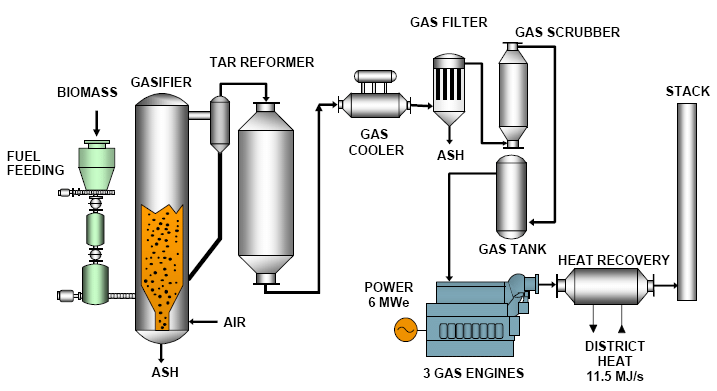
The biomass gasification reaction can be expressed as follows:
C_6H_12O_6 + 6O_2 → 6CO_2 + 6H_2O
In this process, the bacteria ferments the biomass to produce methane and carbon dioxide. The main advantage of this process is that it’s completely carbon-neutral. The main disadvantage is that it requires much land to grow the necessary bacteria.
The first biomass gasification demonstration plant was built in the 1970s by the Swedish power company Vattenfall. The plant used bacteria to convert biomass into methane and carbon dioxide. The plant was decommissioned in the early 1990s. In 2006, Vattenfall started a project to design and build a new biosyngas demonstration plant. This plant uses bacteria to convert biomass into methane and carbon dioxide.
Methane Pyrolysis
Methane pyrolysis is a process in which methane is converted into hydrogen and carbon. The hydrogen can then be used for other purposes.
The methane pyrolysis reaction can be expressed as:
CH_4 → C + 2H_2
Current Market Players
By some estimates, the market for generating hydrogen will be worth $220 billion by 2028. The ten most prominent companies in the hydrogen generation business are:
1. Linde plc (UK)
2. Air Liquide International S.A. (France)
3. Messer Group GmbH (Germany)
4. Air Products and Chemicals, Inc. (U.S.A.)
5. Reliance Industries, Ltd. (India)
6. Cummins, Inc. (U.S.A.)
7. Parker Hannifin (U.S.A.)
8. Hydrogenics (Canada)
9. McPhy (France)
10. FuelCell Energy (U.S.A.)
The Future of Hydrogen Production
Although hydrogen production in today’s era is fossil fuel-intensive, carbon-neutral processes — primarily water electrolysis (see above) — are expected to become much more common as electrolyzers and renewable energy prices fall. According to Haim Israel, the head of thematic investing and global strategist at Bank of America Securities, “we believe [the costs of] both of these will go down another 60 to 70% before the end of the decade.” By then, Bank of America analysts believe “green hydrogen” will account for 22% of the sources of global energy production, up from today’s 4%. Some analysts expect this transition will necessitate an investment of at least $11 trillion in necessary storage, production, and transportation infrastructure.

According to ETF investment group Defiance, the three most essential companies currently involved in this green hydrogen transition are Linde plc, oil giant Shell, and Air Products and Chemicals:
• Linde has been producing green hydrogen since at least 2012 at more than 80 water electrolysis facilities worldwide. The company has plans to triple its green hydrogen production by 2028. The company is building or upgrading plants in Germany, Austria, and Norway to effect this.
• Petroleum producer Shell has been buying electrolyzers for use at its green hydrogen production facilities in Wesseling, Germany, which began operations in 2021. Shell currently has a 20MW hydrogen plant in Zhangjiakou, China, and it’s contracted to build another electrolysis plant in Rotterdam in the Netherlands by 2026. Shell plans to eventually scale this hydrogen production up to 10 times the current levels.
• Air Products and Chemicals is the world’s biggest supplier of merchant hydrogen (hydrogen traded on the open market). The company has plans to build green hydrogen production facilities in Saudi Arabia and the Southwestern U.S.A. shortly.
Sources
The post Hydrogen as an Energy Source appeared first on DAC Consulting.
Industries We Serve
DAC Consulting Services is proud to work with clients across a diverse range of industries.
Our tailored approach ensures that we meet the specific needs of every sector we serve


Oil & Gas
Supporting upstream, midstream, and downstream operations, we offer
expertise in pipeline disputes, offshore projects, and refinery challenges.


Power Generation
From traditional coal and nuclear facilities to modern renewable energy projects, we provide expert insights and technical support for efficient power generation.


Renewable Energy
As the world transitions to sustainable energy sources, our team helps clients navigate the complexities of solar, wind,
and hydroelectric projects.


Construction
We specialise in large-scale public and private sector projects, including airports, bridges, metro systems, and urban developments.


Manufacturing
Our forensic engineering and dispute resolution services are critical for industrial and manufacturing facilities, ensuring operational continuity and efficiency.


Infrastructure
From transportation systems to public utilities, DAC supports infrastructure
projects that underpin communities and economies.
Get in Contact
We are committed to delivering excellence, ensuring clarity, and supporting your success.
Whether you need forensic engineering expertise, expert witness support, or assistance navigating the complexities of your industry, DAC Consulting Services is here to help.
Request a Consultation
Simply fill in the form below and one of our experienced team will contact you to discuss your requirement.
Contact Us
We will get back to you as soon as possible.
Please try again later.
London (HQ) Office
79a Grapes House
Suite 4, First Floor, Esher
Greater London
United Kingdom
KT10 9QA
Business Hours
- Mon - Fri
- -
- Sat - Sun
- Closed